START UP & IRB SUBMISSIONS
REGULATORY SUPPORT
Through collaboration with PERI's Regulatory Team, you will receive support throughout the study from start-up to close out. With their deep understanding of IRB management and established relationships with prominent IRBs, they ensure that your submissions are handled efficiently and effectively, aligning with your overall study strategy.
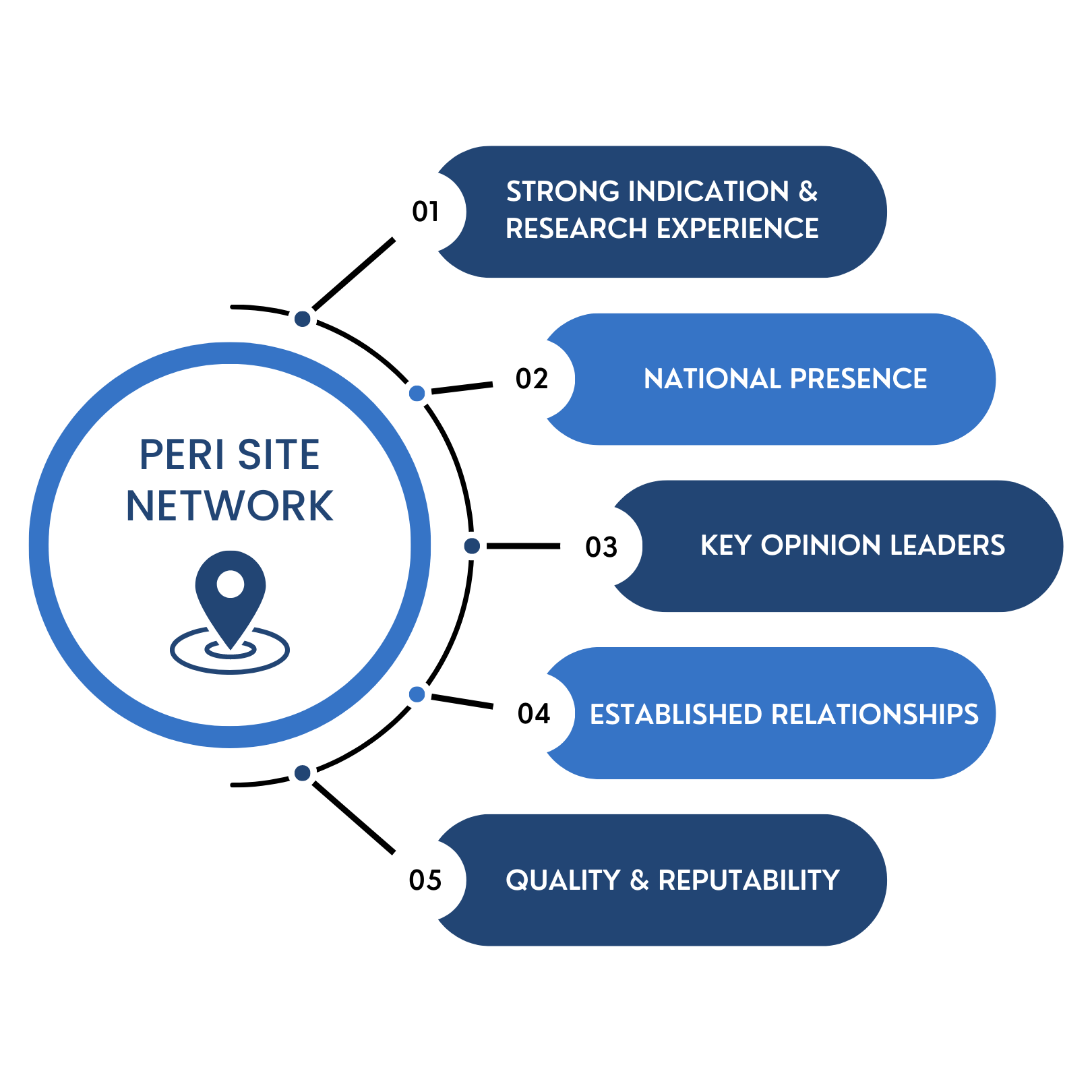
SITE NETWORK
Our streamlined approach to site start-up supports expedited timelines to initiate sites as quickly as four weeks, depending on trial phase. PERI forms strategic partnerships with reputable sites to ensure seamless trial execution and consistent, high-quality subject recruitment.